What Is the Mr of an Ammonia Molecule Nh3
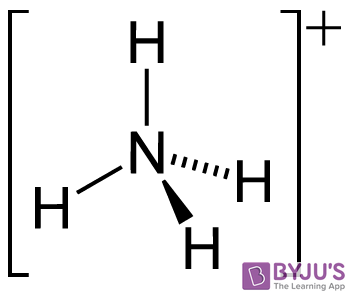
It is the ionised form of ammonia. This type of ammonia is the dangerous part.
NH3 and NH4 together are often referred to as total ammonia.
. But number of atoms can be calculated in this question by multiplying the number of molecules by 4 because there are 4 atoms 1 of N and 3 of H present in every molecule of NH3. Number of moles given mass Mr of the compound. 282 10 26 kg.
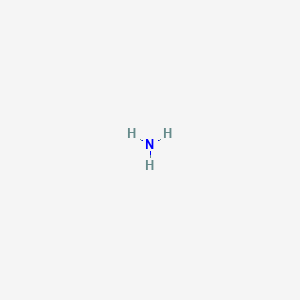
All of the Hydrogen atoms are organized symmetrically across the Nitrogen atom which types the bottom and the 2 nonbonding electrons kind the tip which makes the molecular geometry of NH3 trigonal pyramidal. 4NH3g 5O2g 4NOg 6H2Og What is the limiting reactant if 40 g of NH3 9 2 answers I need help ASAP please. The bond angle in a molecule of ammonia NH3 is 107 levels so why when a part of a transition metallic complicated is the bond angle 1095 levels.
NH3 Molecular Geometry Ammonia has a tetrahedral molecular geometry. Additionally whats the bond angle of ammonia. Convert between NH3 weight and moles.
Molar mass of NH3 1703052 gmol. NH3 molecular weight. Molar mass of NH3ammonia is 1703052 000041 gmol Convert between NH3ammonia weight and moles.
So the molar mass of ammonia is. Molar mass of NH3. NH3 ammonia being a compound would have molecules instead of atoms.
1 Gram Molecular Mass of Ammonia17 ie. To determine the molar mass of any compound all you have to do is add up the molar masses of every atom that makes up the respective compound. Ammonia comprises of one nitrogen atom and three hydrogen atoms.
AmmoniaA few things to consider when finding the molar mass for NH3- make sure you have the correct chemi. 1 mole of ammonia weighs 0017 kg and contains Avogadros number 6023 10 23 of molecules. For historical reasons ammonia is named ammine in the nomenclature.
Since there are 3 Hydrogen atoms present the formula mass of H is 10 3 30 gmol. The molar mass of ammonia will thus be. It exists in two forms in the aquarium and the first step is to understand the difference between ammonium NH4 and free ammonia NH3.
Click to see full answer. NH3 ammonia being a compound would have molecules instead of atoms. Convert grams NH3 to moles or moles NH3 to grams.
BUT the nitrogen atom has 7 protons and each of the hydrogens have 1 proton giving a charge of 10 in total. How many moles in 100grams of ammonia. 17 gmol 1000 g1 kg.
There are 8 electrons in total surrounding the Nitrogen atom. 140067 gmol 3 100794 gmol 1703052 gmol. Ammonia NH3 or H3N CID 222 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
NH4 ammonium is a nontoxic salt. Convert grams Ammonia to moles or moles Ammonia to grams. Mr of NH3 14 1 1 1 17.
Molecular mass of an ammonia molecule 6023 10 23 moleculesmol0017 kgmol. And also in total in the NH3 molecule there are 8 electrons which result in a charge of - 8. Ammonia can act as a ligand in transition metal complexesIt is a pure σ-donor in the middle of the spectrochemical series and shows intermediate hardsoft behaviour see also ECW modelIts relative donor strength toward a series of acids versus other Lewis bases can be illustrated by C-B plots.
Similarly how do you find the mass of ammonia. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride. Compound name is ammonia.
140067 1007943 Percent composition by element. Each NH3 and NH4 ion have SP3 hybridization. In this case you know that ammonia NH_3 is composed of one nitrogen atom N three hydrogen atoms H This means that its molar mass will be the sum of the molar mass of one nitrogen.
Ammonia molecular weight. Track your food intake exercise sleep and meditation for free. So why dosent Ammonia HN3 have an overall charge of 2.
Molar mass of NH3 1703052 gmol. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. Molar mass of NH3 is 1703052 000041 gmol.
140067 1007943 Percent composition by element. Also asked what is the mass of a nh3 molecule. But number of atoms can be calculated in this question by multiplying the number of molecules by 4 because there are 4 atoms 1 of N and 3 of H present in every.
Concerning this whats the distinction between the form of nh3 and nh4 1. Solve any question of Some Basic Concepts of Chemistry with-. Get control of 2022.
Click to see full answer. Enter a chemical formula to calculate its molar mass and elemental composition. A sample of ammonia reacts with oxygen as shown.
This compound is also known as Ammonia. Explanation of how to find the molar mass of NH3. The molecular formula of ammonia is NH3The molecular mass of NH3 is 140 310 170 Amount of NH3 present mass of samplemolar mass 100170 588g.
NH3 ammonia is a gas and sometimes called toxic or free ammonia.

Ammonia Molecular Geometry Hybridization Molecular Weight Molecular Formula Cas Number Bond Pairs Lone Pairs Lewis Structure Infographic
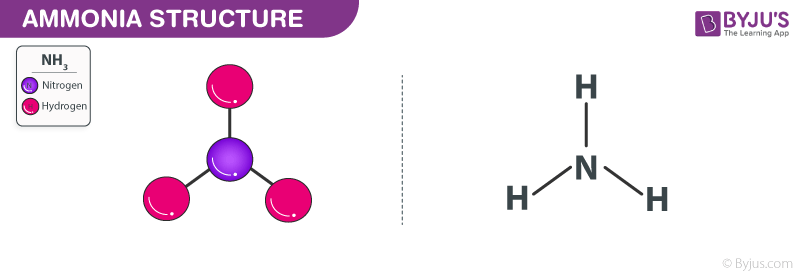
Ammonia Nh3 Ammonia Structure Preparation Properties Uses Of Ammonia

Ammonia Molecular Weight H3n Over 100 Million Chemical Compounds Mol Instincts
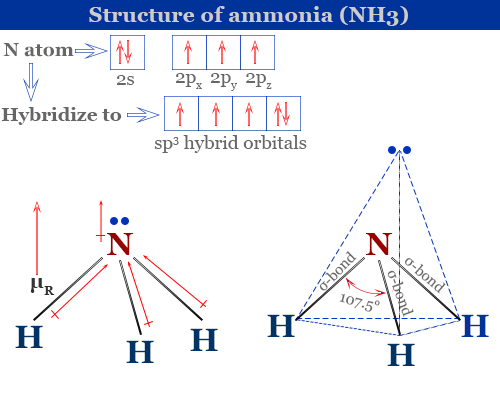
Ammonia Properties Structure Uses Production
What Is Meant By Aqueous Ammonia Solution By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium

Ammonia Molecule Formula Symbol Structure What Is Ammonia Video Lesson Transcript Study Com
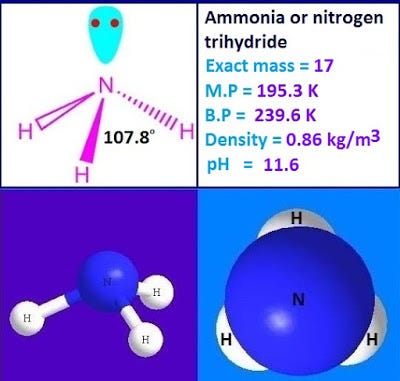
What Is Ammonia Nh3 Ammonia Is A Chemical Compound Of By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium

Molecular Structure Of Ammonia Nh3 Youtube
The Ammonia Molecule With The H N H Angle N H Bond Distance And Download Scientific Diagram

Ammonia Nh3 Ammonia Structure Preparation Properties Uses Of Ammonia

Nh3 Molecular Geometry Shape And Bond Angles Ammonia Youtube
What Is The Total Number Of Atoms In Ammonia Quora
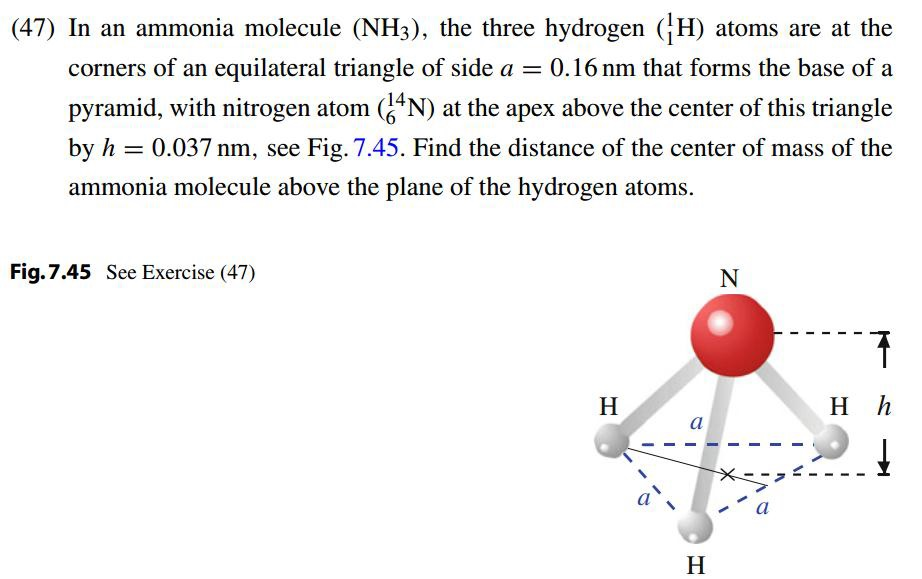
Solved In An Ammonia Molecule Nh 3 The Three Hydrogen Chegg Com

In The Ammonia Nh3 Molecule Of Fig 9 40 Three Hydrogen H Atoms From An Equilateral Triangle With The Center Of The Triangle At Distance D 9 40 10 11

What Is The Molar Mass Of Ammonia Nh3 Youtube

Ammonia Molecule Formula Symbol Structure What Is Ammonia Video Lesson Transcript Study Com
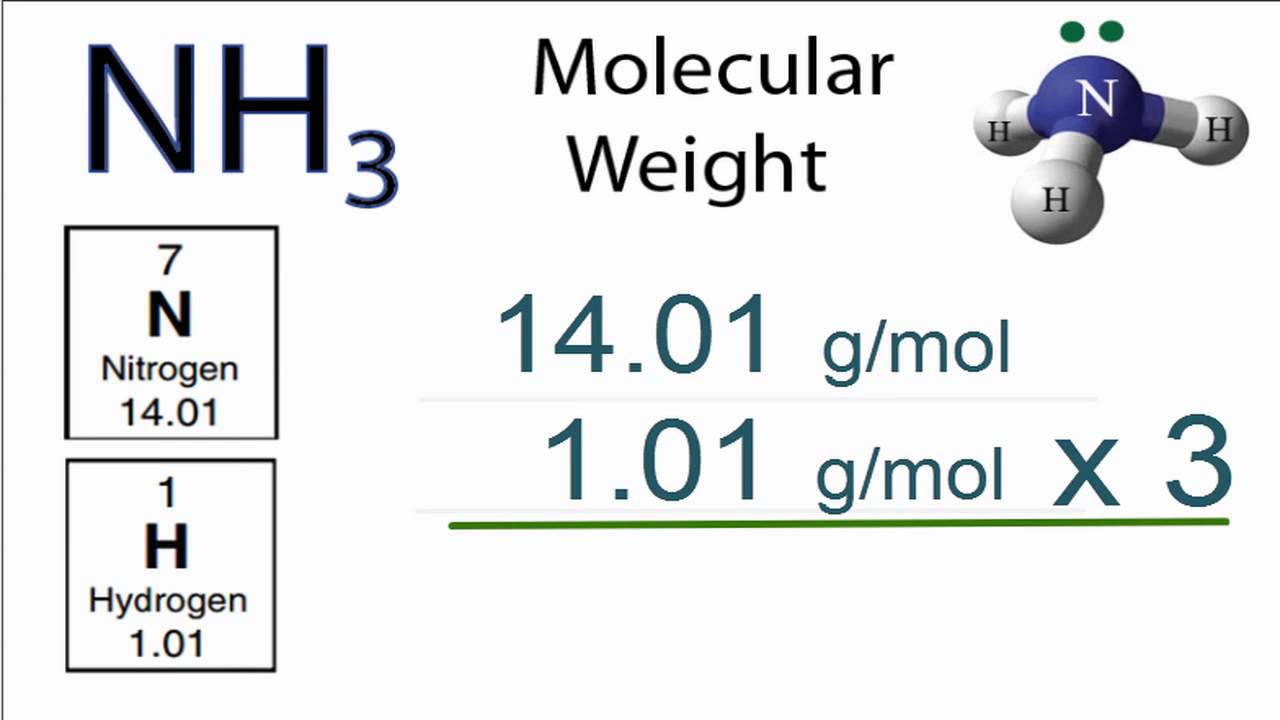
Molar Mass Molecular Weight Of Nh3 Ammonia Youtube
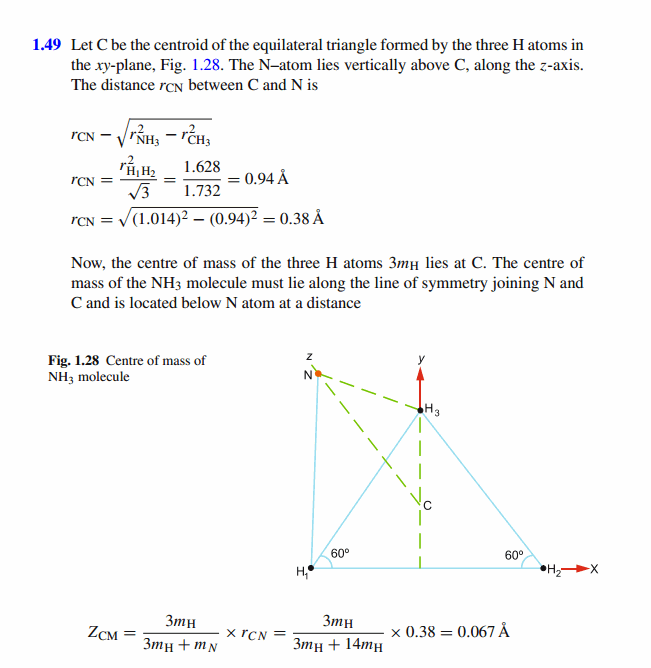
The Ammonia Molecule Nh3 Is In The Form Of A Pyramid With The Three H Atoms At The Corners Of An Equilateral Triangle Base And The N Atom At The Apex Of
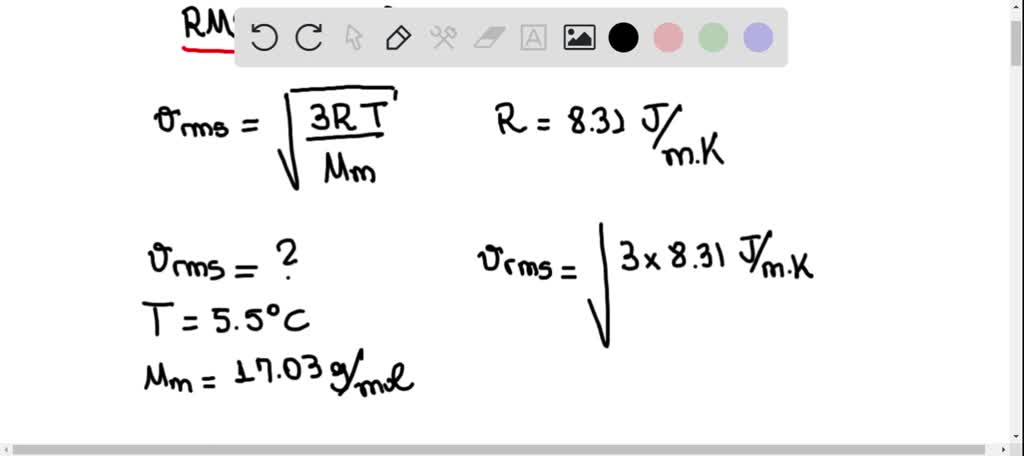
Solved Suppose A Planet Has An Atmosphere Of Pure Ammonia At 5 5 Circ Mathrm C What Is The Rms Speed Of The Ammonia Molecules The Molecular Weight Of Ammonia Mathrm Nh 3 Is 17 03 Mathrm G Mathrm Mol
Comments
Post a Comment