Describe the Construction Working and Useful of Dry Cell
The total voltage of a battery is the sum of all cell voltages. The positive terminal of the battery is projected from the top of this drum.
The terminals of the dry cell are reversed and connected back to the Y-input terminals.
. A rubber bung carrying a thin glass tube with a platinum wire is then inserted taking care that the. This versatility makes it suitable for portable equipment. A chemical reaction strips the hydrogen molecules of their electrons and the atoms become ionized to form H.
Dry cell battery is a voltage-producing battery containing the electrolyte chemical in the form of a thick paste. To obtain greater voltage than the output of a single cell multiple cells must be connected in series. The battery which uses sponge lead and lead peroxide for the conversion of the chemical energy into electrical power such type of battery is called a lead acid battery.
The electrons travel through wires to provide a current to do work. The chemical reactions take place between the zinc container electrolyte and graphite rod. What term is used to describe splitting a large atomic nucleus into two smaller ones.
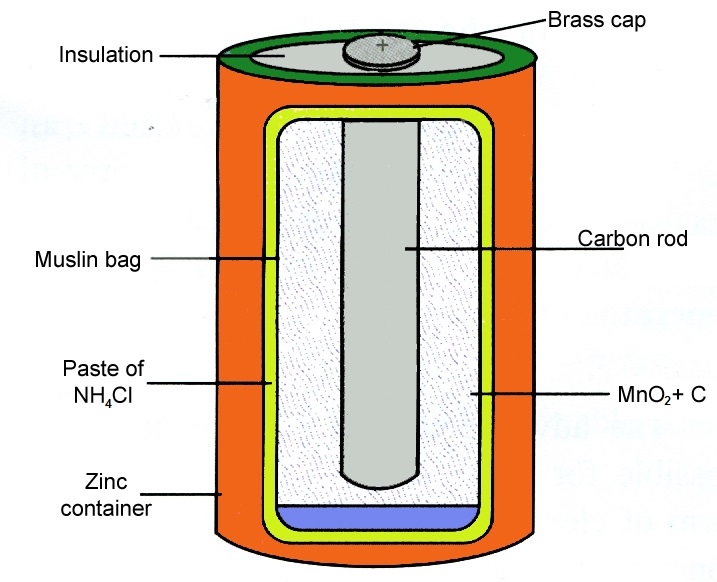
A dry cell consists of a container made up of zinc metal. The electrolyte uses is a moist paste of ammonium chloride. The carbon rod is surrounded by a mixture of carbon particles and a chemical called ammonium chloride.
The oxygen enters at the cathode usually from the air. When the two electrodes of the cell are connected via a closed path then the cell forces the electrons to flow from one end to the other. The Y-gain is set back to 1 V div.
A dry cell consists of a zinc container whose base acts as the negative electrode. It can have positive or negative charge. The size of the cell is irrelevant to its voltage.
The electrode consists of a glass tube provided with a bent side tube and another side tube B. Around this plate there is an outer jacket of glass which has a side inlet through which pure and dry hydrogen gas is bubbled at one atmosphere pressure. The electron flow allows the electrons to flow into the closed system.
Steps 4 to 7 are repeated by using two dry cells connected in series. Diagram of a Dry Cell Battery. A dry cell consists of a metal container in which a low moisture electrolyte paste covers the graphite rod or a metal electrode.
This is surrounded by a mixture of manganese dioxide and charcoal in a muslin bag. Unlike a wet cell a dry cell can operate in any orientation without spilling as it contains no free liquid. Construction of Alkaline Battery.
It produces voltage of about 15 volts. Fine-grained manganese dioxide MnO 2 powder mixed with coal dust is molted to. A typical automotive lead-acid battery has six cells for a nominal voltage output of 6 x 20 or 120 volts.
The position of the bright spot or line on the screen is observed and compared. The dry cell is one of many general types of electrochemical cells. The voltages of the dry cell calculated from the two settings are compared.
The cell pushes the electrons to fluid from one end to the other when the two electrodes are bound in a closed way. According to science an electrode is a conductor used in a battery to run the circuit. A carbon graphite rod with a metal cap serves as a positive terminal.
The lead acid battery is most commonly used in the power stations and substations because it has higher cell voltage and lower cost. These electrodes do not obstruct light to reach the thin p-type layer. Explain the construction and working of a dry cell.
Generally the metal container will be zinc whose base acts as a negative electrode anode and a carbon road acts as a positive electrode cathode. The carbon rod placed at the centre with a brass cap acts as the positive electrode. The container also serves as the negative terminal in the centre.
Due to this reason electric charge is produced or generated on the two terminals positive and negative of the cell and electric current gradually flows into the circuit. A dry cell is a device that generates electricity based on chemical reactions. A dry cell is an electrical power generator dependent on chemical reactions.
Working of a dry cell. This drum contains all materials of the battery and it also serves as the cathode of the battery. 1 Construction.
Description of a dry cell. The flow of electrons causes the current to flow in the closed circuit. A little Hg is placed at the bottom of the dry glass tube.
A solar cell is basically a junction diode although its construction it is little bit different from conventional p-n junction diodesA very thin layer of p-type semiconductor is grown on a relatively thicker n-type semiconductorWe then apply a few finer electrodes on the top of the p-type semiconductor layer. I The standard hydrogen electrode SHE consists of a glass tube at the end of which a piece of platinised platinum foil is attached as shown in Fig. A dry cell has the electrolyte immobilized as a paste with only enough moisture in it to allow current to flow.
The body of the battery is made of a hollow steel drum.

Hho Dry Cell Construction To The Flowbots No Handelbars I Like The Way It Turned Out Dry Cell Energy Machine Hydrogen Generator
Dry Cell Definition Working Principle And Types Of Dry Cell

Intermediate Switch 4 Way Switch Construction Working And Uses Secondary Battery Energy Storage Cell

Doitpoms Tlp Library Batteries Zinc Carbon Batteries Zinc Carbon Dry Cell

A Dry Cell Is One Sort Of Electric Battery Which Is Commonly Utilized For The Home And Versatile Electronic Gadgets Dry Cell Cell Electrochemical Cell

Pin By Peter Holubowski On Concours D Architecture In This Moment Civil Engineering Construction Compressive Strength

Alkaline Batteries Construction Working Of Alkaline Battery Electrical4u

What Is A Dry Cell Battery Find Out Dry Cell Electrochemical Cell Teaching Chemistry

Light Your Way Design Build A Series Circuit Flashlight Activity

Dry Cell Structure Working Chemical Reactions Its Applications

Explain The Construction Of A Dry Cell With An Appropriate Diagram Physics Topperlearning Com 36gl66zz

Dry Cell Structure Working Chemical Reactions Its Applications

Aa Batteries 48count Double A Max Alkaline Battery Packaging May Vary Energizer Battery Energizer Alkaline Battery

Draw Neat And Labelled Diagram Of Dry Cell

Construction Of Zinc Carbon Battery Leclanche Cell Electrical4u

Cell Organelle Flip Book Learning Worksheets Cell Parts Cell Organelles




Comments
Post a Comment